See the answer Which of the following species are isoelectronic with Ne. Check out a sample QA here See Solution Want to see the full answer.

Which Of The Following Species Is Isoelectronic With Ne
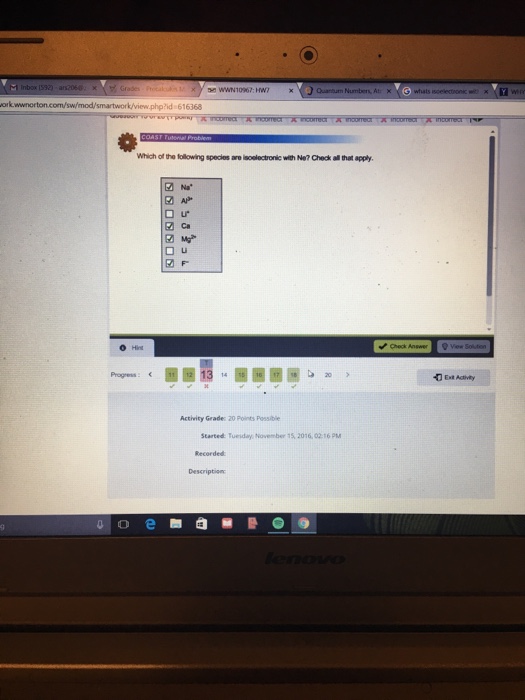
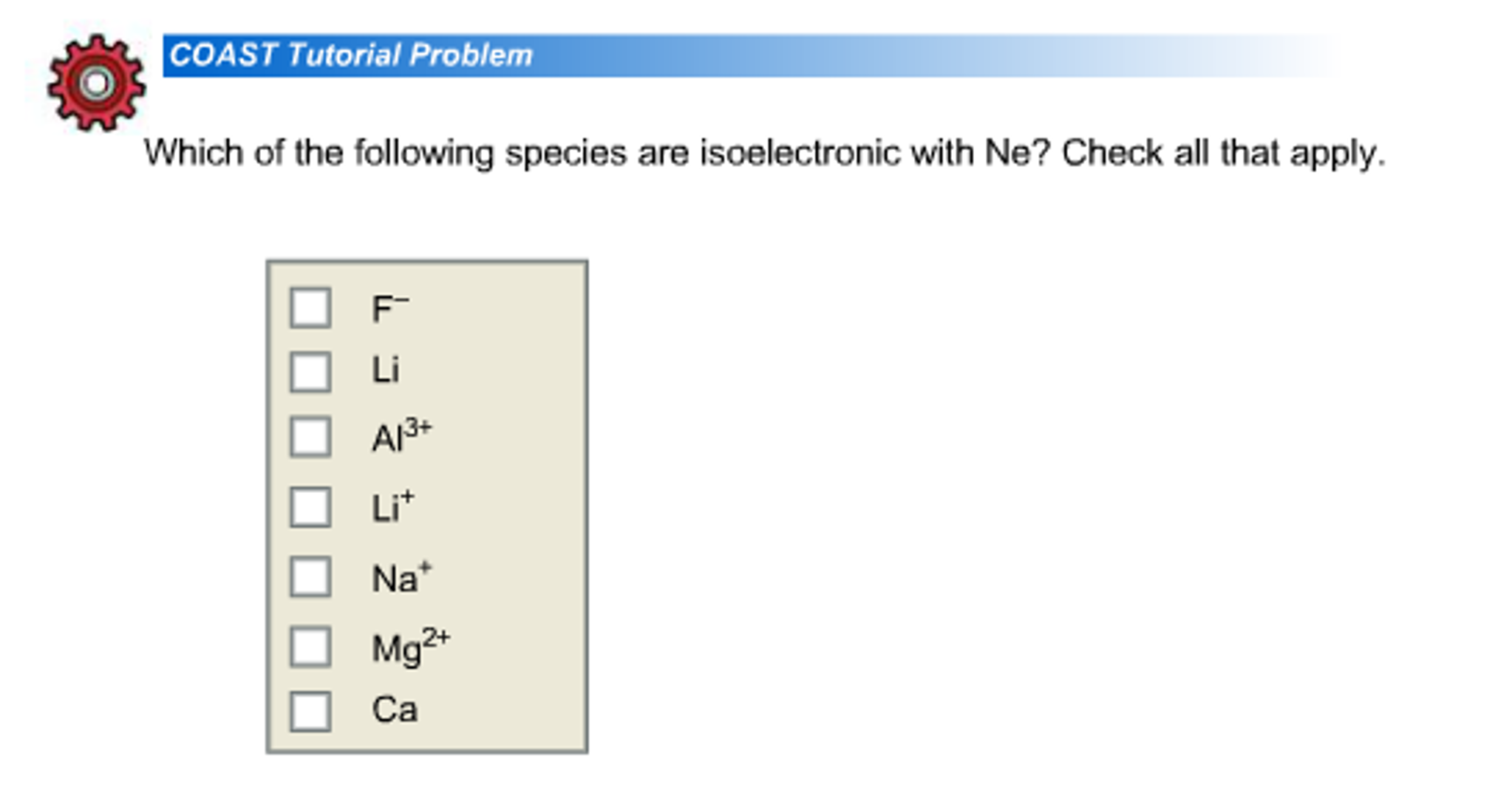
1 Mg2 2 Ca 3 F- 4 Al3 5 Li 6 Li 7 Na Expert Solution Want to see the full answer.

. Question Which of the following species can NOT function as a reducing agent. O 8 Ma E. When the 2 electrons are taken away from the calcium ion it has 18 electrons and when 2 electrrons are added to the selenium ion it has 36 electrons.
Which of the following species are isoelectronic with Ne. 0 0 Find All solutions for this book. Explanations Question Arrange the following species in isoelectronic pairs.
Recent Posts Latest PMC MDCAT 2022 date Pakistan May 12 2022. View Solution Related Answers Question a. Which pair is isoelectronic with each other.
Mg2 Ca Li Al3 F- Na Li Expert Answer 100 19 ratings Previous question Next question. Which of the following elements are smallest and why. Explanation Reveal next step Reveal all steps.
Mg2 Ca Li Al3 F- Na Li Answer Concepts and reason The concept used to solve this problem is to determine the Iso-electronic species with neon on the basis of. K and Ar Two species are isoelectronic if they have the same number of electrons. Which of the following species are isoelectronic with Ne.
Hence Ne O 2- Na are isoelectronic species. A calcium atom has 20 electrons and a selenium atom has 34 electrons. Li Na K C.
Which of the following species are Isoelectronic with Ne. Mathrm O O mathrm Ar Ar mathrm S 2- S2 mathrm Ne Ne mathrm Zn Zn mathrm Cs Cs mathrm N 3- N3 mathrm As 3 As3 mathrm N N mathrm Xe Xe. Na K Mg 2 Ca 2S 2-Ar Which of the following species are Isoelectronic with Ne.
Li Mg2 Li Na F- Ca Al3 Solution 5 1 Ratings Solved Chemistry2 Years Ago74 Views This Question has Been Answered. Check all that apply. S 21s 22s 22p 63s 23p 6 Ar1s 22s 22p 63s 23p 6 K 1s 22s 22p 63s 23p 6 Ca 21s 22s 22p 63s 23p 6 ArS 2Ca 2andK are isoelectronic species.
Check all that applymg2CaLiAl3F-NaLi This problem has been solved. Choose one or more. Expert Answer 100 4 ratings Transcribed image text.
Which of the following are isoelectronic species. Video Explanation Was this answer helpful. Which of the following species are isoelectronic with Ne.
Which of the following species is isoelectronic with Ne. Mg2 Ca Li Al3 F-Na Li. Two neutral elements cannot be isoelectronic so the correct pair must be either an element and an ion or two ions.
Which of the following species are isoelectronic with Ne. Question Which of the following species are isoelectronic with Ne. All of the following species are isoelectronic except Aft folowing species are isoelectronic A.
H H H- B. Na Mg 2 are isoelectronic species. QA which of the following species are isoelectronic with Ne.
1 Answer Prev Question Next Question Find MCQs Mock Test. Another isoelectronic series is P3 S2 Cl Ar K Ca2 and Sc3 Ne3s23p6. 1Answer 1vote answeredJan 22 2020by Rubby01503kpoints selectedJan 22 2020by Pankaj01 Best answer All elements contains total 10 electrons.
Maharashtra State Board HSC Science Computer Science 11th. N 3-Na Al 3ArRb F - 380727556 32 k 97 k 0346. Answer Concepts and reason.
They contain 18 electrons each. Check all that apply. They contain 10 electrons each.
The concept used to solve this problem is to determine the Iso-electronic species with neon on the basis of total number of electrons in the given species Fundamentals. Ca² K¹ Ar Cl. Of Ne 10.
Identify from the following the isoelectronic species. Asked Sep 26 2020 in Periodic Classification of Elements by Manish01 477k points Which one of the following is isoelectronic with Ne. C1- Br- I D.
Textbook Solutions 6916 Important Solutions 17. Check all that apply. CSS Essay Paper 2022 May 12 2022 12th Class 2nd Year Date Sheet 2022 Lahore Board May 12 2022 MOPA Ministry of Parliamentary Affairs Jobs 2022 May 12 2022 Online 9th Class Roll Number Slip 2022 Bise Lahore Board May 12 2022.
Na Mg2 Al3 Ca F- Li Li 1 Answer isoelectronic means having the same electron configuration same of electrons usually after becoming an ion. Atomic no of Ne10. N3- Na Al3 Ar Rb F- class-11 Share It On FacebookTwitterEmail Please log inor registerto add a comment.
Why are Ca² and Se² not isoelectronic. A N3- b Mg2 c Al3 d All the above periodic classification of elements class-11 Please log in or register to answer this question. Ne O2- Na OR Ar Cl2- K.
Check all that apply. Check out a sample QA here See Solution star_border. Isotopes Isobars and Isotones.
Solved Which Of The Following Species Are Isoelectronic With Chegg Com

Solved Which Of The Following Species Are Isoelectronic With Chegg Com
Solved Which Of The Following Species Are Isoelectronic With Chegg Com

Solved 23 Which Of The Following Species Is Isoelectronic Chegg Com
0 Comments